Preclinical research and neural signal stability
Preclinical research and neural signal stability
Preclinical research and neural signal stability
Proven longevity and decoding accuracy with Connexus® BCI
This blog was updated from 2.5 to 3 years of stable recordings on October 1, 2025.
Recent news headlines on brain-computer interfaces (BCIs) focus on exciting early results, including short-term follow-up in human clinical trials and animal studies demonstrating new capabilities. These early successes fuel optimism and showcase the potential of emerging technologies in healthcare innovation.
However, ask anyone who might consider neurosurgery to receive a BCI, and one priority quickly emerges: long-term reliability. Patients want a device that serves them for years, ideally for the rest of their lives. This means that BCIs, like all implanted medical devices, must withstand the harsh biological environment of the body, operate reliably over time, and minimize the need for a risky replacement surgery.
In a previous blog, Neurotech that lasts, we described the core principles behind producing a high-data-rate brain implant that is capable of delivering long-term performance. In this article, we share data from our preclinical research demonstrating over two years of reliable neural recordings that strengthen the foundation for translational research and clinical adoption of the Connexus® BCI.
Key findings from preclinical research in BCI
- We have conducted safety and functional testing in the sheep auditory cortex (hearing part of the brain).
- In over 3 years of data collection from our R&D electrode array, we demonstrated our ability to record stable neural signals over time, with practically no degradation in signal-to-noise ratio, and sustained decoding accuracy over time.
- In functional testing of the fully-implanted Connexus® BCI up to 26 weeks, we have measured similarly high signal-to-noise ratio and even better decoding performance compared to the R&D device.
Animal testing as a foundation for translational research
At Paradromics, we perform our safety and functional testing in sheep using an acoustic stimulus paradigm. Compared to the human brain, sheep have similar brain folding (i.e., sulci and gyri) and cortical architecture; thus sheep are commonly used in translational research (John et al. 2017; Opie et al. 2018) and in preclinical research of neuromodulation devices. The sheep auditory cortex in particular accommodates the placement of our cortical module in a configuration representative of human-use implantation, while also supporting our surgical technique and instrument development.
As will be the case in human implantation, cortical modules are placed subdurally, directly on the surface of the sheep brain. This results in hundreds of thin microelectrodes (40 micron in diameter) extending 1.5 mm into the auditory cortex from which neural signals are recorded. Furthermore, because the electrodes are placed in the auditory cortex, by playing acoustic stimuli (i.e., pure audible tones) for the sheep, we can observe the stimulus-driven response in the neural recordings as the sounds are heard.
Below we present two sets of animal study results: first, a 3-year follow-up of our R&D testing of the Connexus electrode array, and second, the six-month results for the on-going prospective functional animal study of the full Connexus BCI.
Long-term preclinical research on the Connexus electrode array
To assess the Connexus electrode array in vivo, we implanted two sheep with a cortical module representative of the final finished device. This experimental system provides periodic neural signal recording over the lifetime of the device implantation, allowing us to assess long-term neural recording performance. Through this over 3-year follow-up period (beyond 1000 days post-implantation, with data collection going), we have observed no electrode array migration, the sheep are healthy, the recorded neural signals remain stable, and we continue to successfully decode neural responses to stimuli. This extended preclinical research period provides strong translational research findings to support clinical trials.
Neural signal features identified in preclinical BCI testing
The figure below shows data collected 1,112 and 1,084 days post-implantation for Sheep 1 and 2, respectively. For each sheep, we show example output traces for four channels from the cortical module. These traces have been filtered to focus on the neural spiking band (i.e., the frequency band used for decoding neural activity). Each spike represents a neuron firing; for each spike that reached a certain threshold, we made a tick mark. The pattern of tick marks across time are referred to as a raster plot and form a set of threshold crossing features. These features, measured for each channel simultaneously, are then input into the data analysis pipeline for neural decoding.
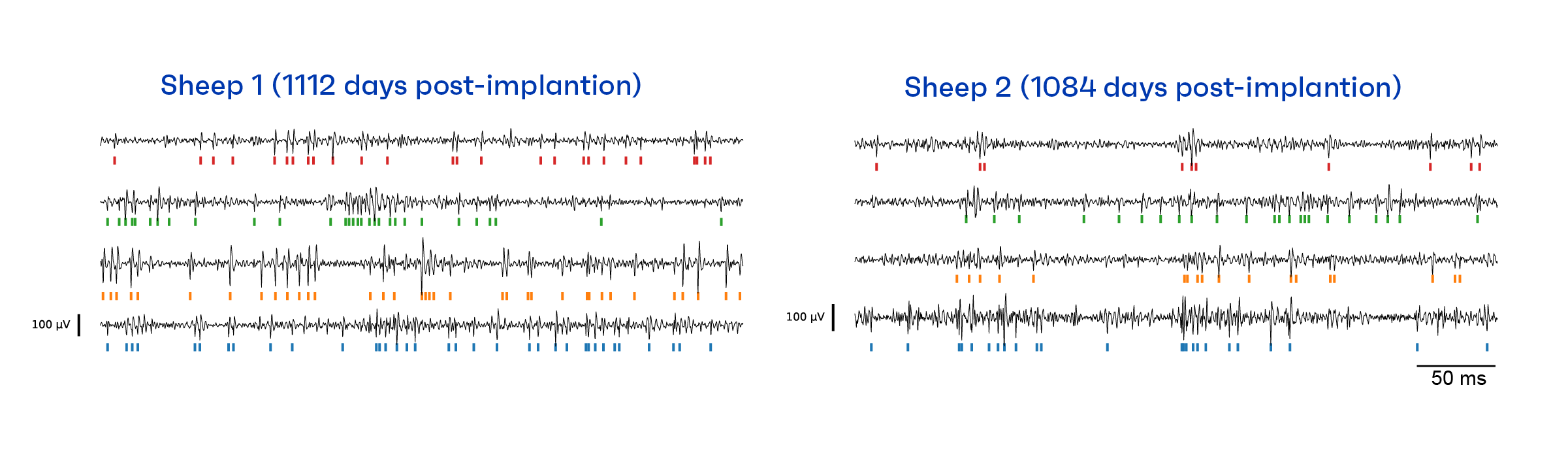
Assessing neural recording stability through preclinical research
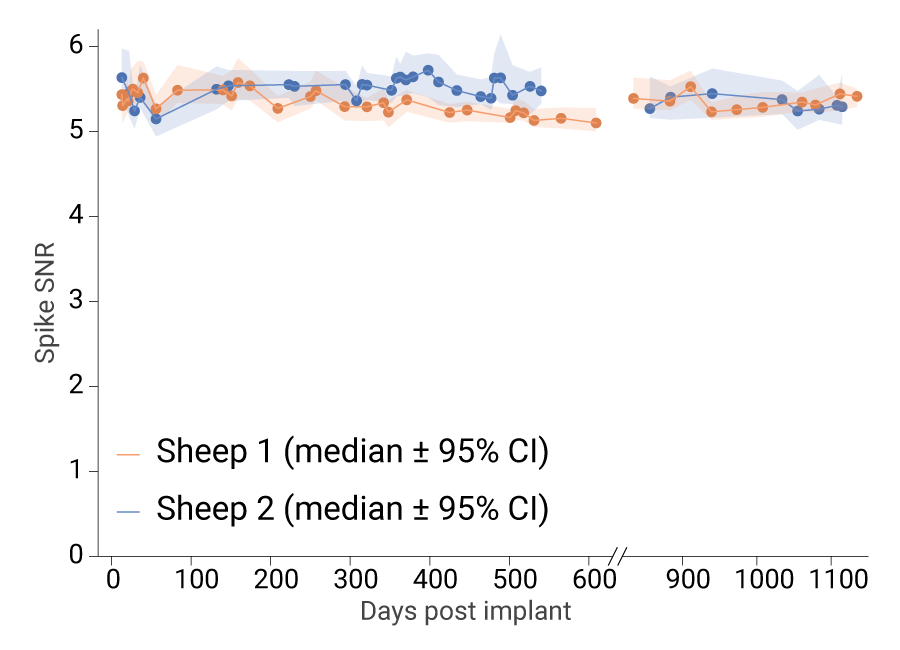
To this day, we continue to record robust spiking activity in these animals, and the spike signal-to-noise ratio (SNR) across both sheep has reached a stable plateau. Spike SNR is a measure of signal quality; the higher the ratio, the better the signal quality.) This high SNR (>5) for our cortical module recordings in sheep is as good or better than those published for chronically implanted Utah Arrays in the rhesus macaque motor cortex, which ranged from 1.5 to 4 (Sponheim et al. 2021). The Utah Array is a research device that has enabled high-performance BCIs [Willett, et al. 2023; Card, et al. 2024; Willsey, et al. 2025;Singer-Clark, et al. 2025]; however, it is known for its highly variable functional lifespan.
Note that at about 600 days post-implant, the ongoing recordings were paused to focus on a new cohort of animals for the Connexus BCI prospective study, with recordings resuming at about 800 days, resulting in a gap in the data.
Decoding stimulus responses to advance translational BCI research
In addition to analysing the raw neural signal output, we studied the ability to record and decode the neurophysiological response to stimuli. To do this, we played brief acoustic tones of distinct frequencies for the sheep and recorded the stimulus-driven spiking activity from hundreds of channels simultaneously. Then, we developed a decoding algorithm to identify the frequency of each tone stimulus based on the recorded neural activity.
Within about 10-15 ms after the tone begins playing, many of the auditory cortex neurons fire more vigorously than their baseline activity. While many of the electrode channels appear to have correlated activity, not all channels behave in the same way. We leverage the data patterns across electrode channels and time for these stimulus-driven neural responses to develop effective decoders.
The figures below are data visualizations of a neural latent space. In machine learning, a latent space represents complex data in a more compact form to make patterns and features easier to identify and learn. These loop-shaped trajectories represent the stimulus-driven neural response for the 150 ms after the tone begins. On the top left, the individual temporal trajectories are plotted for 4 different sound frequencies with 30 trials per sound. Each of these trajectories is generated by mapping the higher dimensional neural recording from hundreds of individual electrode channels to the three dimensional latent space shown. The plot on the right represents the average neural response across trials for each stimulus condition. The animated plot on the bottom shows how these trajectories develop over the 150 ms of the neural response to the sound. Our decoder leverages the consistent structure of these neural trajectories in latent space to identify the stimulus tone.

Visualizing stimulus response. Each stimulus trial condition (sound frequency) is color coded. The latent neural traces occupy different parts of the latent space, i.e. the colors are separated in this space. As expected, the sham control condition, in which no stimulus is played, shows much less variability than the stimulus conditions.
Sustained performance in long-term preclinical research
To further assess the reliability of the Connexus electrode array, we measured decoding accuracy and mutual information over time. That is, if we can accurately determine which tone was played based on the recorded neural signals, then the device continues to function effectively. Similarly, mutual information (MI) is the measure of how much useful information the recording signal carries about the tones. The figure below shows that over time we continue to extract meaningful information, with both decoding MI and accuracy remaining relatively stable beyond the 3 years of data collection. These preclinical research results are a critical step for translational research studies developing reliable high-performance BCI technology.
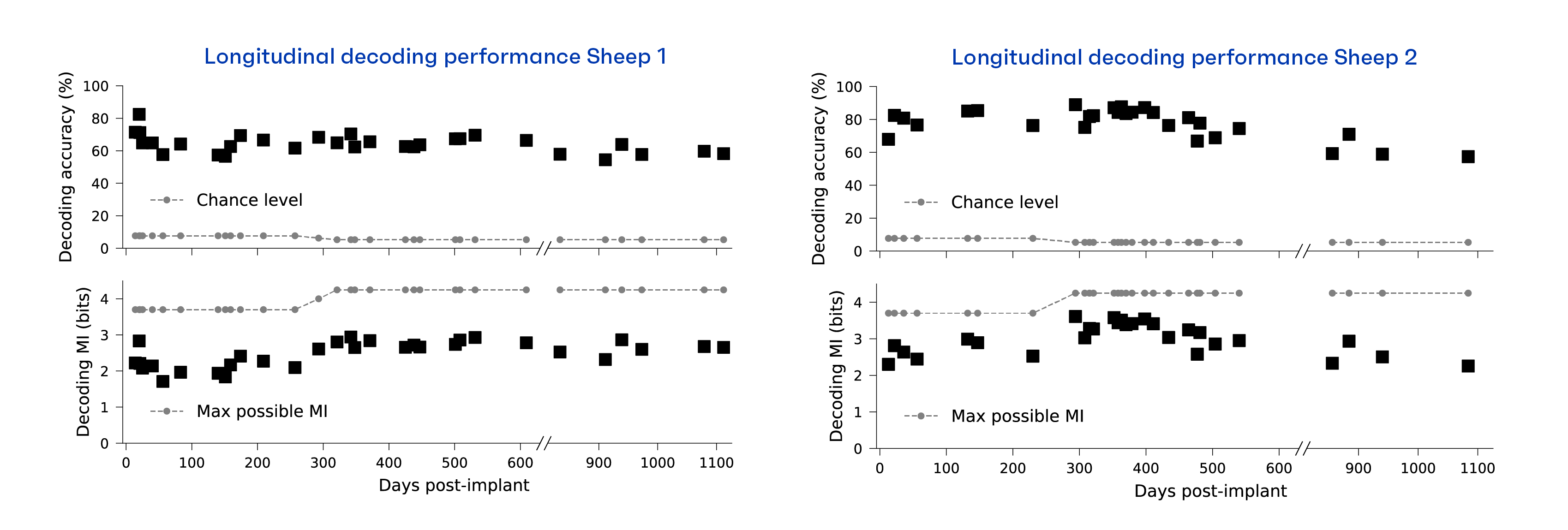
Early translational research results from the Connexus BCI study
Adding to the robust results from the Connexus electrode array, we also evaluated the neural recording stability and decoding accuracy of the fully implantable system, consisting of the Connexus Cortical Module, Extension Lead, and Internal Transceiver. This preclinical research was conducted over a 26-week study period with over 6,100 active device-hours. Verifying results with the fully implantable system is an important part of preclinical research verification and validation testing to evaluate the device’s intended use.
Identifying spiking features in the neural data
We conducted long-term awake neural recordings in six implanted sheep (Sheep A–F) over the study period. The signal quality across all animals was comparable to that observed in Sheep 1 and 2 from the R&D electrode array testing. Representative output traces from all six sheep are shown below, illustrating distinct spike events.

Assessing performance of neural recording over time
Longitudinal spike SNR remained stable above 4, consistent with the signal quality observed in Sheep 1 and 2. These results demonstrate the repeatability and robustness of the device performance across multiple animals.
.png)
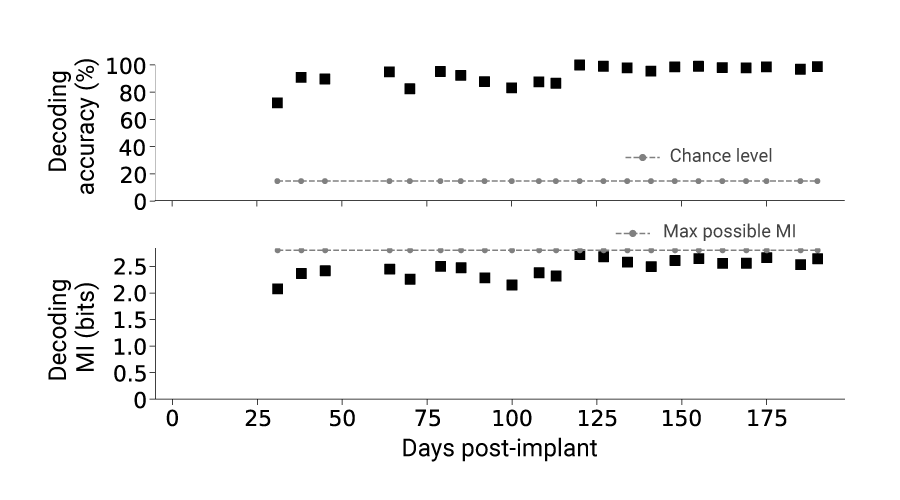
Assessing decoding performance over time
Additionally, throughout the 26-week period, we were able to extract meaningful information based on decoding mutual information (MI), with decoding accuracy consistently well above chance for the entire time across all 6 sheep. In fact, decoding performance improved compared to Sheep 1 and 2, which were implanted with a Connexus electrode array. The figure below illustrates an example of tone decoding performance for Sheep C over time.
Conclusion: Preclinical research validating robust BCI performance
These results demonstrate stable, high-quality signal recording over time and provide compelling evidence that the Connexus Cortical Module can reliably capture key neural features over extended periods of implantation. This capability is critical for long-term use of high-performance intracortical BCI applications like speech decoding [Willett, et al. 2023; Card, et al. 2024] and control of computer devices [Willsey, et al. 2025, Singer-Clark, et al. 2025, Nuyujukian, et al. 2018]. These preclinical research results and translational research findings demonstrate that the Connexus BCI is reliable and information-rich, meeting key requirements for advanced applications such as speech decoding.
If you have any questions, please reach out to media@paradromics.com.
%20(1).svg)

