Electrocorticography (ECoG) surveys the landscape
Electrocorticography (ECoG) surveys the landscape
Electrocorticography (ECoG) surveys the landscape
Intracortical electrodes mine for gold
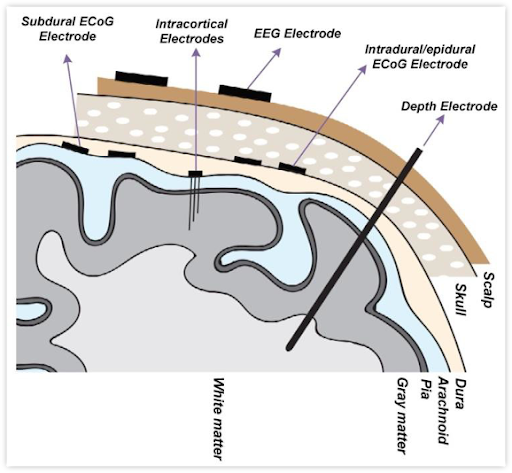
What is the difference between surface electrodes and intracortical electrodes?
When it comes to brain recording, the first rule of real estate applies: Location. Location. Location. Some applications of this rule are obvious, i.e. if you are building a vision system, record from the visual cortex and not the motor cortex. However, there is another important aspect of location that is sometimes overlooked in neurotechnology: proximity to signal.
There are critical differences between recording brain signals from outside the skull, on top of the brain, or inside the brain. The comparative advantages of these approaches lie at the intersection of the resolution of data provided and what surgical risks are involved in their placement and removal. In this blog, we unpack some of the advantages and disadvantages of two different approaches to implantable brain-computer interfaces (BCIs): brain surface recording (electrocorticography or ECoG) and recording using microelectrodes that penetrate into the brain, usually 1-2 mm deep (intracortical).
Surface recording plays a clinical role in surgical mapping and diagnostics.
ECoG involves the surgical placement of electrode grids or strips directly onto the exposed cortical surface of the brain. ECoG has a long and well-established history in clinical neurology, particularly in neurosurgical contexts. Its primary clinical applications include epilepsy focused localization and intraoperative cortical mapping of areas that support motor function, sensory function, and language, helping to preserve these areas during epilepsy surgery.
An advantage of ECoG recording over techniques that penetrate the brain surface is that the electrodes can be easily placed, removed, and repositioned mid-surgery. ECoG also captures signals across large areas of the brain surface, but this carries the disadvantage of requiring a larger craniotomy. Overall, ECoG is useful for clinical diagnostics, where ease of deployment outweighs optimization for signal quality or chronic stability.
Surface recording has its limitations.
At the surface of the brain, ECoG electrodes are unable to capture the spiking activity of individual neurons, instead measuring the summed activity of hundreds of thousands of neurons across a few millimeters of cortex. These electrical fluctuations primarily come from the coordinated activity of neurons across cortical layers (depending on frequency, timing, and other factors). These signals, known as local field potentials (LFPs), capture the broader rhythms that surround the more detailed and information rich activity of individual neurons (e.g. isolated spikes, spike bursts, and correlated spiking patterns across space and time) (see Figure 1).
.png)
While ECoG captures cortical signals that can be employed in basic research studies to examine the neural correlates of behavior (e.g. physical movement, speech amplitude changes, and changes in sensation), there are challenges due to the distance of the electrodes from individual neurons, the greater noise corruption, and the averaging of underlying sources.
ECoG signals have been used in Brain-Computer Interfaces (BCIs), where the signals have been converted into computer-driven outputs like cursor movement, mouse clicking, letter generation, or movement of a robotic arm. However, when it comes to industry leading BCI applications, ECoG falls short in translating brain signals into outputs that approximate the flexibility and speed of natural human behavior.
In the case of reproducing human speech, for example, sophisticated decoding models have been employed in attempts to further reduce delays in ECoG decoding, but the speech outputs still show multi-second delays. These delays are far from the decoding speeds demonstrated using signals that record from individual neurons. In addition, even when ECoG covers ~100x of the brain’s surface area (compared to intracortical electrodes), the output capabilities of ECoG are still substantially reduced (e.g. producing highly constrained vocabularies limited to about 50 to 1000 different words).
Electrocorticography has the potential for wider brain-state estimation, but limited value for low latency BCI feedback and control.
ECoG grids allow the identification and functional assessment of a large area of cortex (i.e. they help “survey the landscape”). However, this coverage carries the disadvantage of requiring a large craniotomy. Another disadvantage is the lack of unique information available through ECoG cortical signals compared to the information available using intracortical electrodes placed directly next to neurons.
While the use of ECoG for BCI neurotech is much less widespread than its clinical history, there have been some studies that have evaluated its potential capabilities in a variety of motor and communication tasks, including cursor and prosthetic control. Broadly, these studies demonstrate that some amount of control is possible, but that the level of control achieved falls far below that of able bodied individuals, as discussed in more detail below. This makes clear that electrocorticography alone cannot deliver the richness of signal needed to truly restore natural abilities for BCI patients.
Before comparing ECoG and intracortical electrode BCI findings, it is important to note that the percent accuracy levels reported can vary widely based on the details of the BCI interface output. There is often a tradeoff where higher accuracy levels may be reported with a limited range of outputs, meaning that the results become less biologically relevant despite high reported accuracies. In addition, accuracy levels may be artificially inflated by making targets larger or reducing the number of response options.
This nuance has been appreciated for intracortical electrode BCI studies going back to 2012 and 2013, with Collinger et. al. 2013 generating more realistic and relevant movement evaluations using clinically relevant stroke evaluation methods. Relatedly, Gilja et. al. 2012 explicitly compared monkey cursor control with performance of the animals own arm.
Biologically relevant metrics are the gold standard of BCI neurotechnology applications if we are serious about returning full independence to those without the ability to move or speak.
Many electrocorticography BCI applications have focused on movement, including controlling prosthetic arm movements, driving exoskeletons to assist with walking for tetraplegic patients and generating certain hand gestures. For example, a 2017 study used ECoG signals from the sensorimotor cortex of epileptic patients to decode hand gestures like "scissors," "rock," and "paper," with accuracies between 69.7% and 85.7%. Another study was able to decode between four gestures with 97% and 74% accuracy. But the output “vocabulary” of these studies is low, even down to two options (partial hand grips or whole hand grips), and in many cases only the simple onset or offset of speech or movement is decoded.
It is also important to note that it is standard to observe multi-second delays between motor intent and decoding within ECoG BCI interface studies. The limited availability of data often means that the window of analysis spans multiple seconds, as in the Bleichner et. al. 2016 study referenced earlier. An intracortical electrode array, in contrast, could provide a substantial reduction in decoding delays (i.e. on the order of ~100-200 milliseconds) while additionally generating a greater range of movements and higher decoding accuracies. For those with limited movement and speech, these improvements in timing, accuracy and range of movement can make a life changing difference.
The richest signals come from the neurons themselves.
Intracortical electrodes provide at least a tenfold increase in information rate and a tenfold decrease in decoding delay (compared to electrocorticography).
The initial ECoG BCI interface findings suggest a limited “upper range” of movement capabilities beyond a handful of gestures, calling for a need to search for more detailed measures of brain activity at the level of single neurons. Since the electrical signal falls off quickly at small distances, capturing the activity of single neurons requires electrodes right next to neurons, as in the case of intracortical electrode neurotechnology. Intracortical electrodes are often designed to reach 1.5 mm below the surface of the cortex (e.g. Paradromics Connexus® device) where they can measure the strong spiking activity of cortical processes that are projected out to the rest of the brain (Figure 2).
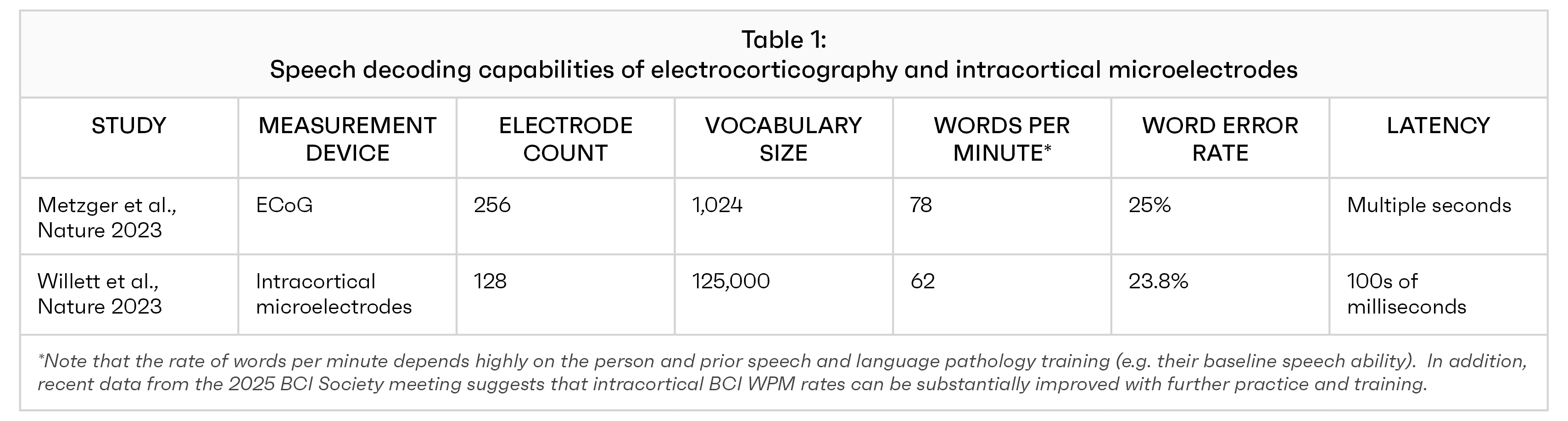
Two papers published in the same issue of Nature (2023) clearly illustrate that intracortical electrodes substantially outperform ECoG, especially in complicated tasks like speech decoding. The Metzger et al. ECOG study produced 78 words per minute with a 1,024 word vocabulary, a 25% error rate, and a multiple second latency. In contrast, the Willett et al. microelectrode study produced 62 words per minute with a 125,000 word vocabulary, a 23.8% error rate, and latencies around 100s of milliseconds.
While the error rates and number of words produced were somewhat similar across the two studies, the higher vocabulary and lower delay of intracortical electrodes is a critical finding, since it approaches the vocabulary and delay of adult human speech abilities (see Table 1). Meanwhile, the 1,024 vocabulary of the ECoG study matches the typical number of words recognized by a three year old child.
The possibilities of BCI speech decoding have been advanced even further by Card et. al. in 2024, using twice as many electrodes (256 vs. 128) and arrays (4 vs 2) as the Willett et al. study. After training, they were able to achieve 97.5% accuracy with a 125,000 word vocabulary and a word error rate of 2.5% over the course of 8.4 months of use. While the communication rate was around 32 words per minute, the system was able to predict phonemes every 80 milliseconds and became the participants preferred method of communication.
It is also worth noting the reduced training time of this study compared to Willett et. al., as well as accuracy levels that surpassed smartphone dictation levels and approached that of able-bodied speakers reading aloud (1-2% error rate). When it comes to synthesizing voice in real-time, this sort of low latency BCI decoding is critical, along with the ability to decode paralinguistic activities like changes in intonation and word emphasis, providing even stronger support for the life changing opportunity of intracortical electrode BCIs.
ECoG’s performance ceiling: Why surface-level signals can’t match intracortical resolution
ECoG remains constrained in its potential for widespread, high-performance BCI-use due to inherent technical and physiological limitations. The fundamental limitations arise from its spatial averaging of cortical activity, capturing neural signals that are summed together rather than discrete single-unit spikes. This “smeared” signal limits the resolution and information content, reducing its ability to decode fine-grained motor outputs, such as individual finger movements, or to achieve naturalistic, fluid speech.
“Like a flashlight in the dark, ECoG helps spot the brain’s most vital regions, paving the way for higher-resolution tools, like intracortical microelectrodes, to unlock the underlying patterns that create speech and movement.”
Given these limitations, ECoG's enduring strength lies not necessarily in being a standalone high-performance BCI for complex tasks, but rather in its role as a "flashlight" or mapping tool in advance of more detailed measures. It excels at identifying specific cortical regions, thereby guiding the placement of more powerful measurement techniques like intracortical microelectrode arrays for higher-performance brain-machine interfaces. In addition, ECoG's ability to measure activity across larger areas of cortex means it will continue to be valuable in guiding neurosurgery.
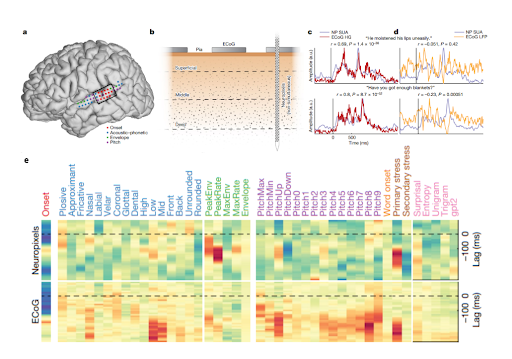
The location and role of the brain area is also an important consideration when it comes to comparing the information available from ECoG and microelectrode recordings. A comparison between the two signals in the same subjects indicates that high frequency ECoG recordings closely track single unit activities across the layers of the cortex (Figure 3, c). In early sensory areas, neural activity more closely follows the qualities of the stimulus input and thus high frequency ECoG also tends to track these features.
However, as stimulus processing gets more complex further along the brain's processing hierarchy, a more detailed distribution of neural spike codes begins to emerge, and ECoG recordings miss out on this valuable information (Figure 3, e). This sentiment is captured by the following quote from Duraivel et. al. 2023: “Across the depth of cortex, the neuronal population is tuned to a dominant speech feature, consistent with the high-frequency broadband signal recorded at the surface with ECoG. At the same time, a relatively large proportion of neurons throughout the vertical cortical column also encode a large variety of other speech features, revealing a distinct, previously unappreciated dimension for speech encoding.”
Fundamentally, the difference between ECoG and intracortical array neurotechnology lies in their proximity to the location of information processing in the brain. Intracortical electrodes sit beside the neurons that shape and share the brain’s most essential information. ECoG, conversely, records aggregate LFP signals that represent a more macroscopic, blurred average of a large number of neurons. Compared to intracortical electrodes, ECoG lacks access to the detailed, high-frequency spiking patterns that underlie the precise coding of sensory features and the production of fine movements and rapid speech.
ECoG technology is innovating, but the physical limitations will always remain.
Within the past few decades, there has been a trend toward using microfabrication techniques and novel materials to produce ECoG arrays with higher density and superior material properties. Some of the emerging leaders in micro-ECoG technology include NeuroOne, Precision Neuroscience, INBRAIN, and Neurosoft Bioelectronics.
The movement of the field toward modern material and manufacturing techniques could have important implications for both device cost and longevity, but it will not substantially change the amount of data that can be extracted from the brain surface. Micro-ECoG is ultimately constrained by the same “law of diminishing returns,” where the data becomes more and more redundant as the electrodes get closer and closer together.
For example, IRB approved academic studies have pushed the limits of electrode density even further than devices designed for commercialization, demonstrating there is little value in pushing electrode densities past submillimeter electrode spacings. In the case of the Precision Neuroscience device, their claim of safely delivering the device through a small burr hole or skull slit also needs to be critically evaluated, since the established method for ECoG electrode deployment is through a craniotomy-based procedure.
Conclusion: The limited future uses of electrocorticography
ECoG has contributed to advancements in research and possesses a strong clinical history. However, its primary utility will not be in BCIs, but as an advanced monitoring and mapping tool in clinical practice, allowing for functional localization during surgery and long-term monitoring for conditions like epilepsy.
ECoG-based BCI interfaces have comparatively reduced utility in terms of performance, with the majority of applications focusing on low dimensional tasks like slow and imprecise 2D cursor control, simple selection, the opening and closing of a robotic hand, or binary (yes/no) communication. These applications provide modest quality of life improvements, but truly lifechanging BCI applications require intracortical arrays with greater accuracy, a greater vocabulary or flexibility of movement, and shorter delays.
ECoG has reached a plateau in terms of data density and will face challenges expanding to the high-performance BCI neurotech space. Nevertheless, it holds onto a solid historical foundation as a valuable and complementary neurosurgical tool, continuing to augment clinical practice and provide valuable research insights, despite not serving as the enabling “gold standard” for BCI performance.
If you have any questions, please reach out to media@paradromics.com.
%20(1).svg)

